Details of the Drug
General Information of Drug (ID: DM2LJFZ)
| Drug Name |
4-hydroxy-2-nonenal
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Hydroxynonenal; 4-Hydroxy-2-nonenal; 4-hydroxynon-2-enal; trans-4-Hydroxy-2-nonenal; 4-Hydroxy-2,3-nonenal; 4-HNE; 75899-68-2; 4-hydroxy-2E-nonenal; (E)-4-hydroxynon-2-enal; CCRIS 9028; CCRIS 1781; 29343-52-0; 128946-65-6; 2-NONENAL, 4-HYDROXY-; HNE; (E)-4-hydroxy-2-nonenal; (2E)-4-hydroxynon-2-enal; (2E)-4-Hydroxy-2-nonenal; CHEMBL454280; CHEBI:58968; C9H16O2; (+/-)4-HYDROXYNON-2-ENAL; NCGC00161254-02; 4-hydroxynonen-2-al; CHEBI:32585; CCRIS 6927; 4 hydroxynonenal; (E)-4-Hydroxynonenal; AC1Q2VOQ; 4HNE; SCHEMBL3920; AC1Q6PP5; AC1NR22M
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
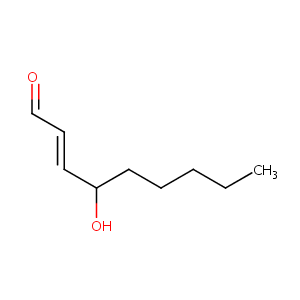 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 156.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
